The world, in all its mysteries, is filled with scientific phenomenon that challenges the way we build, conduct business, and innovate.
Galvanic corrosion is one such electrochemical phenomenon that structural engineers must consider in their work.
Although potentially damaging, galvanic corrosion is easily preventable with the right materials, design, and engineering to guide you there.
In this article, we’ll cover:
- What is galvanic corrosion?
- What does galvanic corrosion damage look like?
- How to prevent galvanic corrosion?
Follow along this helpful guide to learn more and find the right solution for your needs. 💡
What is Galvanic Corrosion?
Galvanic corrosion is an electrochemical process where different metals or alloys in direct contact with one another develop a corrosion potential when exposed to an electrolyte, such as water.
Also known as dissimilar metal corrosion, this phenomenon enables the metal that is more resistant to oxidization (noble metal) to act as a cathode to the other (active metal).
However, like fire, galvanic corrosion occurs only when all three of these conditions are present:
- The metals have different corrosion potentials
- There is direct metal-to-metal contact
- A conductive electrolyte regularly connects the two metals
If any of these conditions are not present, then galvanic corrosion does not and will not occur.
Next, let’s take a look at some common examples of galvanic corrosion, and how to identify when it’s a problem. 👇
Damage from Galvanic Corrosion
If you spend just a bit of time wandering about, you are sure to find examples of galvanic corrosion in your own environment.
The most common form of this phenomenon, is, of course, rust on iron.
However, galvanic corrosion occurs between all different kinds of metals, especially when there are larger variations in conductivity.
Corrosion commonly occurs with steel and aluminum fittings, or steel and brass. Here is a comprehensive list of metals and their properties.
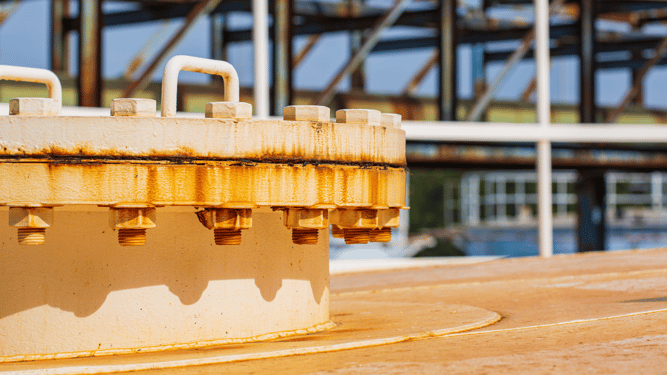
Galvanic corrosion weakens the strength and integrity of the metal. For structures and buildings, this can lead to long term structural issues if the corrosion progresses.
READ NEXT: ["What is the Purpose of Vibration Isolation Pads?"]
How Dissimilar Metals Are Affected
Take a look at the list of dissimilar metals. The noble metals, like graphite and platinum, are more conductive than their active counterparts.
If you were to pair stainless steel with aluminum alloys, there is a greater chance for galvanic corrosion to take place.
There are examples of galvanic corrosion everywhere, simply because there are many types of metals used across industries.
Water is the most abundant electrolyte. Whether it be steam, rain, or submersion, there is more potential for galvanic corrosion anytime these metals are connected in any environment where water is present.
Since it is so common, dissimilar metal corrosion is not always a big issue.
However, if you are in an industry where reliability and durability are a must, like construction or manufacturing, galvanic corrosion causes significant damage to structures, machines, and other connecting parts.
How to Prevent Galvanic Corrosion
Luckily, galvanic corrosion prevention is typically quite easy to design and install.
Like we mentioned before, all three conditions must be met for galvanic corrosion to take place between dissimilar metals.
Therefore, all that is required to prevent galvanic corrosion is a tough, resilient piece of material to separate the dissimilar metals.
The picture above features one example of how Fabreeka designs for galvanic corrosion prevention. Our signature vibration isolation materials are some of the toughest on the market.
Fabreeka® Pad
Trusted by manufacturing, building, and industrial professionals for over 100 years, our signature cotton-duck pad stands the test of time as one of the most reliable bearing pads on the market.
Perfectly suited to prevent galvanic corrosion, the Fabreeka pad breaks metal-to-metal contact while being impermeable to most oils, water, mildew, steam, and brine.
LEARN MORE: ["What is Fabreeka Pad?"]
SA-47 ROF Pad
For a cost-effective, sustainable solution, look no further than Fabreeka’s SA-47 random oriented fiber pad.
Made from a blend of recycled rubber compounds, the SA-47 pad has nearly all the compressive strength of the Fabreeka Pad with the same resiliency and resistance to liquids.
The SA-47 pad is cut and designed much like our Fabreeka pad, and is exceptionally suited as a long-term solution to prevent galvanic corrosion.


-png.png?width=367&height=550&name=Add%20a%20heading%20(1)-png.png)




SUBMIT YOUR COMMENT